The entropy of vaporisation of water at 100^∘ C, if molar heat of vaporisation is 9710 cal mol ^-1 will
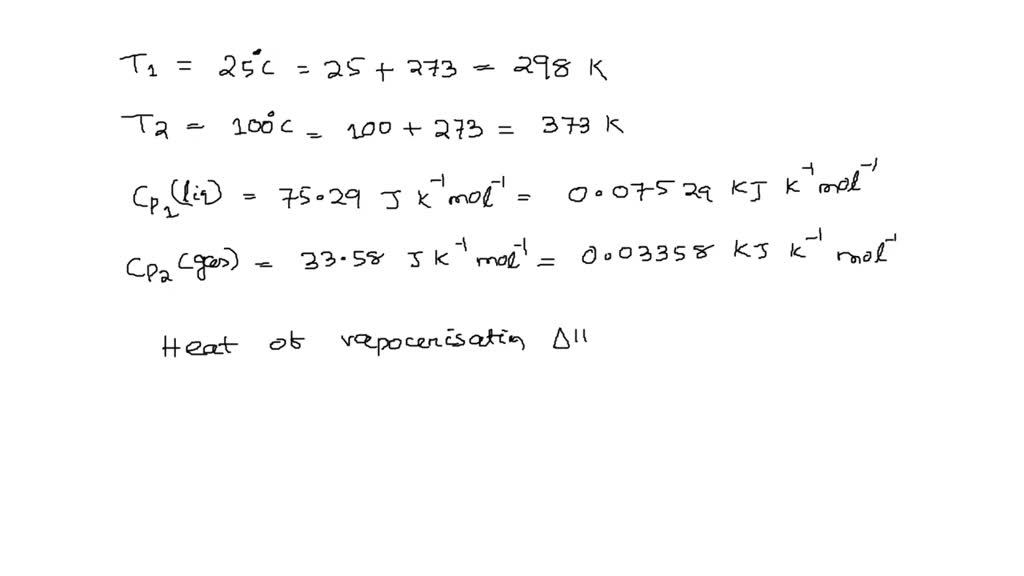
SOLVED: Estimate the enthalpy of vaporization of water at 100 C from its value at 25 C, 44.01 kJ mol-1, given the constant pressure molar heat capacities of 75.29 J K-1 mol -

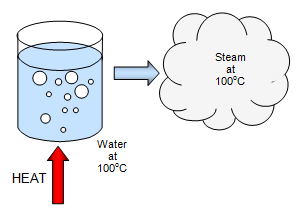
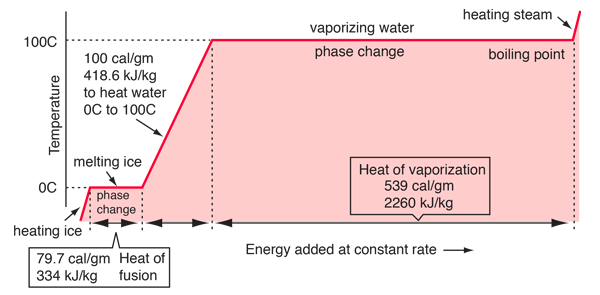
SOLVED: When 1.0kg of steam at 100*€ condenses to water at 100*C. what is the change in entropy of the steam? The latent heat of vaporization of water is 22.6 x 10

The enthalpy of vaporization of water at 100^o C is 40.63 KJ mol^-1 . The value Δ E for this process would be:
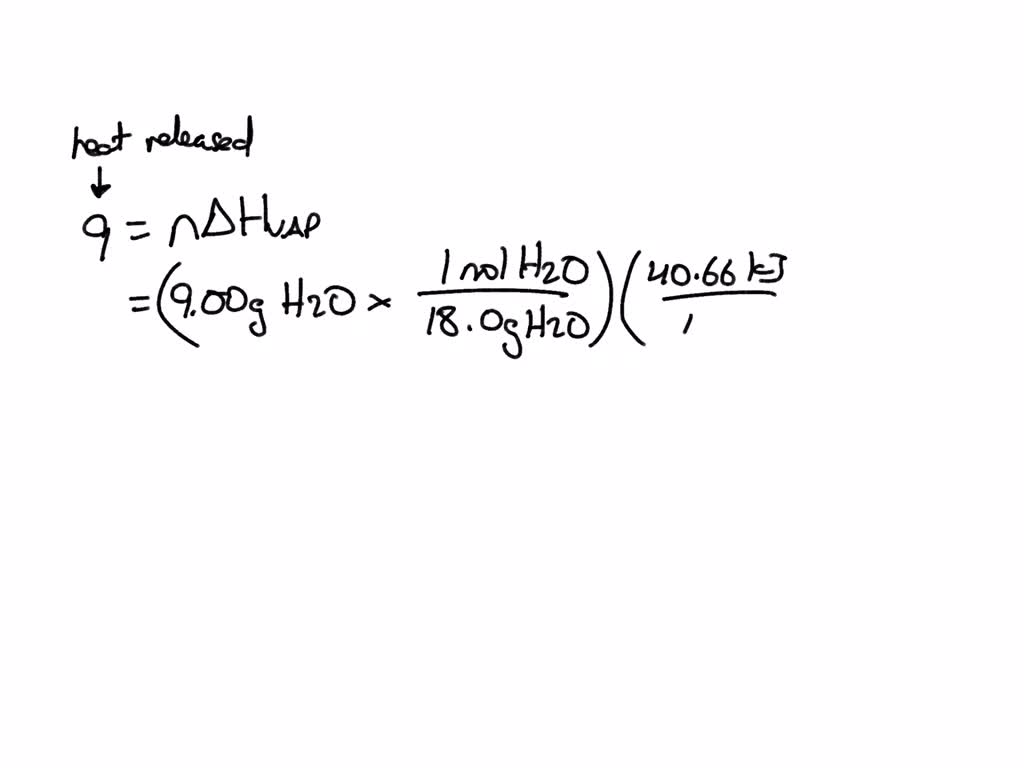
SOLVED: the heat of vaporization of water at 100*c is 40.66 kj/mol. calculate the quantity of heat that is absorbed/released when 9.00 g of steam condenses to liquid water at 100*c

Calculate the entropy change for vaporization of `1mol` of liquid water to stem at `100^()C`, if... - YouTube

The enthalpy of vaporisation of water at 100^∘C is 40.63 kJ mol^-1 . The value Δ E for this process would be .
19 The latent heat of vaporisation of water at 100 degree Celsius is 540 cal/g. Calculate the entropy increase when one mole of water at 100 degree Celsius evaporated 1) 26 cal/k