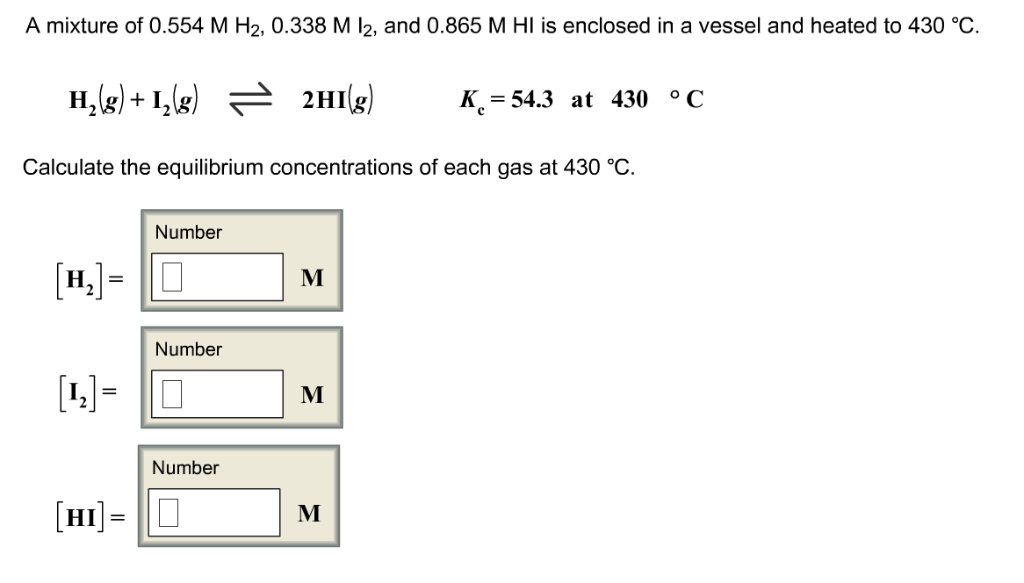
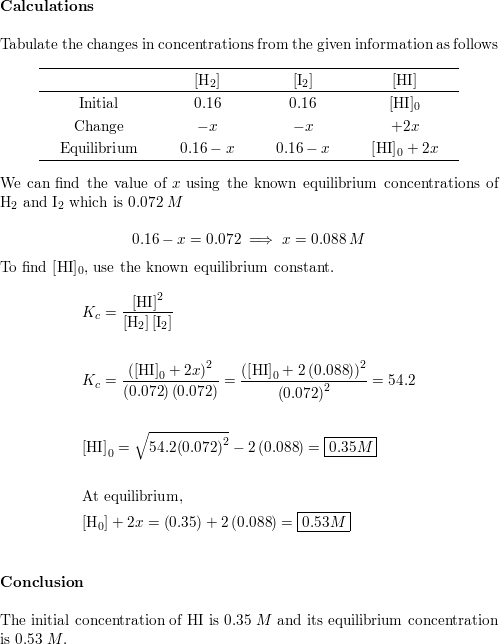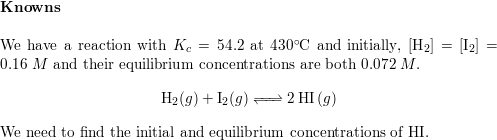Part 1: Calculating Equilibrium Constants 1. A mixture of H 2 and I2 is allowed to react at 448°C. When equilibrium is establi

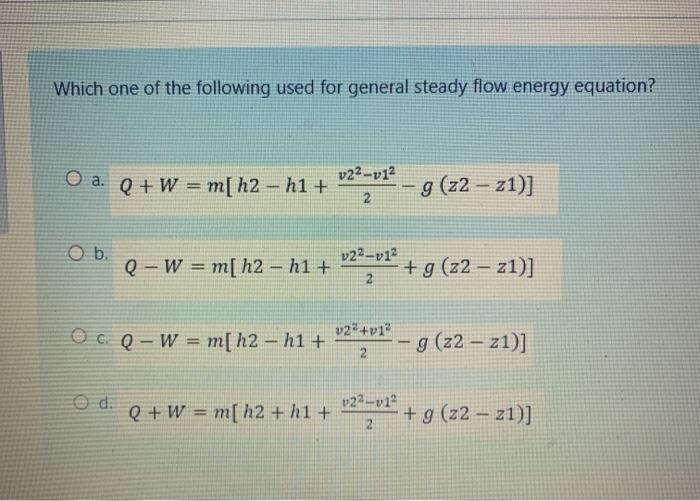
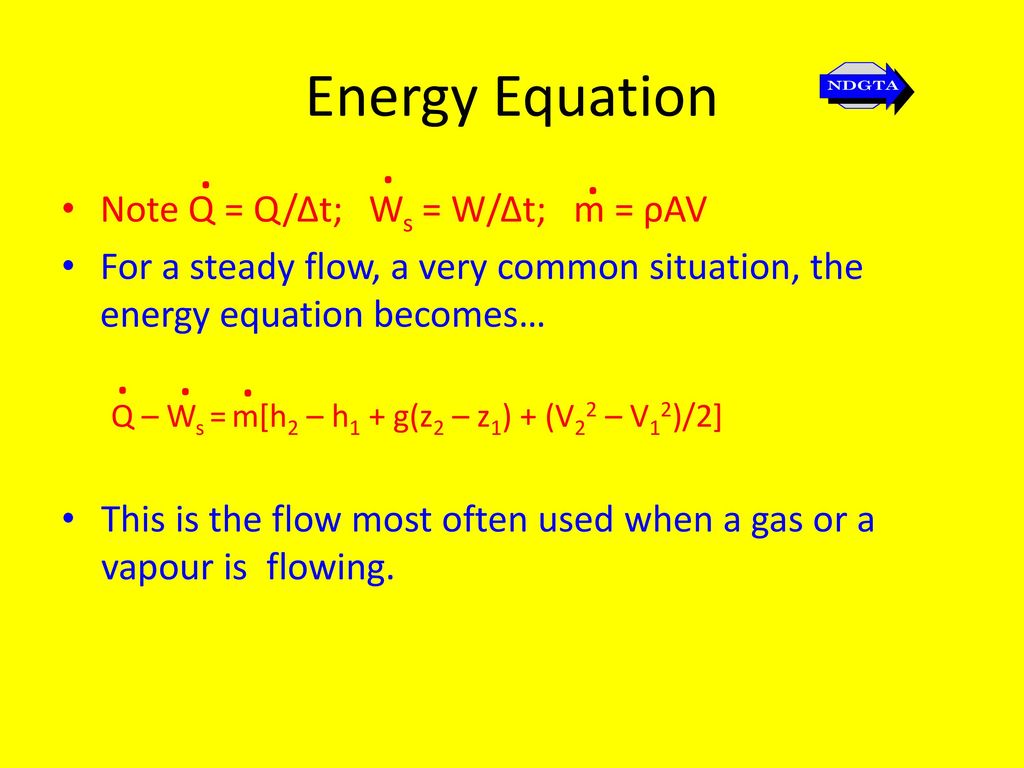
The steady flow energy equation:Q = m(h2 – h1) is applicable for:a)Nozzleb)Turbinec)Compressord)BoilerCorrect answer is option 'D'. Can you explain this answer? | EduRev SSC JE Question

queueing theory - M/H2/1 Queue - Explicit Expression for Response Time Distribution - Mathematics Stack Exchange

13.82 | The equilibrium constant (Kc) for this reaction is 1.60 at 990 °C: H2(g) + CO2(g) ⇌ H2O(g) - YouTube
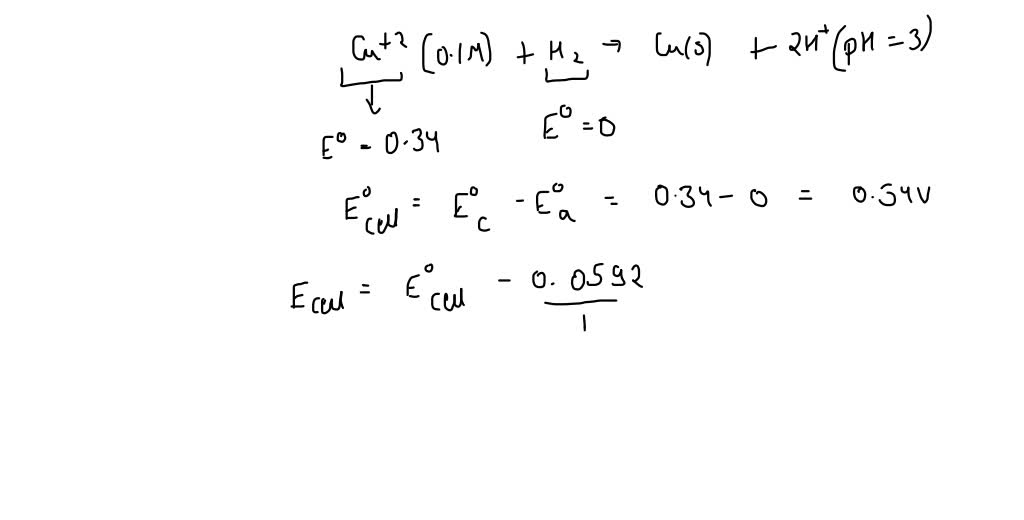
SOLVED: Calculate the cell emf for the following reaction: Cu2+(0.10 M) + H2(1 atm) ® Cu(s) + 2H+(pH = 3.00) A) 0.49 V B) 0.19 V C) 0.15 V D) 0.40 V E) –0.34 V
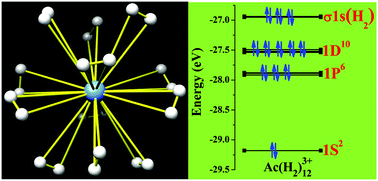
Predicted M(H2)12n+ (M = Ac, Th, Pa, U, La and n = 3, 4) complexes with twenty-four hydrogen atoms bound to the metal ion - Chemical Communications (RSC Publishing)
![SOLVED: Consider the reaction: CO(g)+H2O(g)⇌CO2(g)+H2(g) Kc=102 at 500 K A reaction mixture initially contains 0.130 M COand 0.130 M H2O. Part A What will be the equilibrium concentration of [CO]? [CO] = . SOLVED: Consider the reaction: CO(g)+H2O(g)⇌CO2(g)+H2(g) Kc=102 at 500 K A reaction mixture initially contains 0.130 M COand 0.130 M H2O. Part A What will be the equilibrium concentration of [CO]? [CO] = .](https://cdn.numerade.com/ask_previews/36628956-c06f-439b-80ff-a0f936ba3b67_large.jpg)
SOLVED: Consider the reaction: CO(g)+H2O(g)⇌CO2(g)+H2(g) Kc=102 at 500 K A reaction mixture initially contains 0.130 M COand 0.130 M H2O. Part A What will be the equilibrium concentration of [CO]? [CO] = .
![Calculate Kp for the reaction, C(s) + H2O(g) CO(g) + H2(g) at 990 K if the equilibrium concentration are as follows : [H2O] = 1.10 M, [CO] = [H2] = 0.2 M, Calculate Kp for the reaction, C(s) + H2O(g) CO(g) + H2(g) at 990 K if the equilibrium concentration are as follows : [H2O] = 1.10 M, [CO] = [H2] = 0.2 M,](https://dwes9vv9u0550.cloudfront.net/images/6653152/88b9752c-8929-4745-bc8f-8705cb2ad9b9.jpg)