Half life of a first order reaction is 2.1xx10^(12)s. Calculate the rate constant of the reactio... - YouTube

The reaction `A(g)toB(g)+2C(g)` is a first order reaction with rate constant `2.772xx10^(-3)sec^(-1) - YouTube

Chemical Kinetics Class 12 Notes Chemistry Chapter 4 - Learn CBSE | PDF | Reaction Rate | Reaction Rate Constant

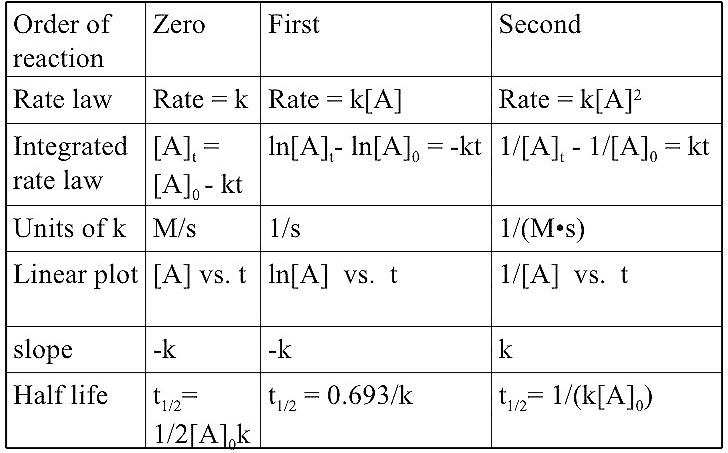
SOLVED:The following data have been obtained for the decomposition of N2 O5(g) at 67^∘ C according to the reaction 2 N2 O5(g) →4 NO2(g)+O2(g) Identify the order of the reaction with respect

A first order reaction is found to have a rate constant k= 5.5 xx 10^(-14)s^(-1). Find half-life of the reaction.
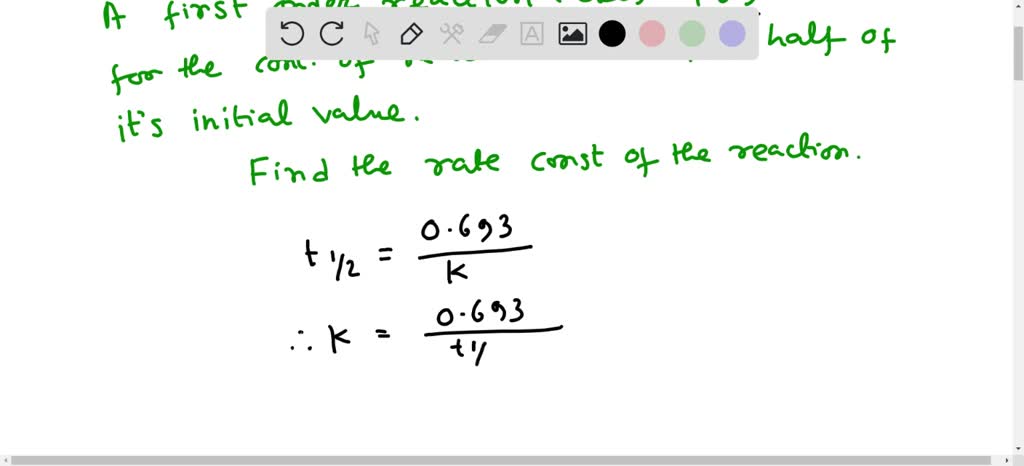
A first order reaction completes 50% at the end of 50 minutes. What is the value of rate constant in sec^-1? How many times will the reaction be complete at 87.5%? - Quora
SOLVED: A reaction occurs by second-order kinetics. If it takes 130 seconds for the concentration of reactant to decrease 1.3 M to 0.017M, what is the value of the rate constant (k)

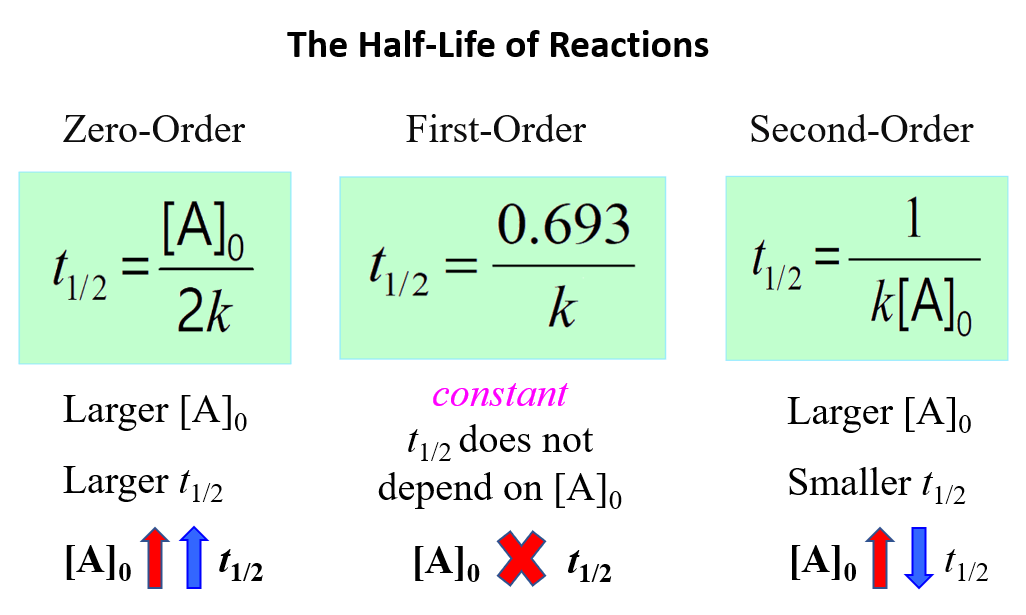
Calculate the half life of a first order reaction from their rate constants given below:(a) 200 s^-1 ; (b) 2 min^-1 ; (c) 4 year^-1 .

Define half life of a reaction Derive the relationship between half life and rate constant for a first Order - Chemistry - - 16068871 | Meritnation.com
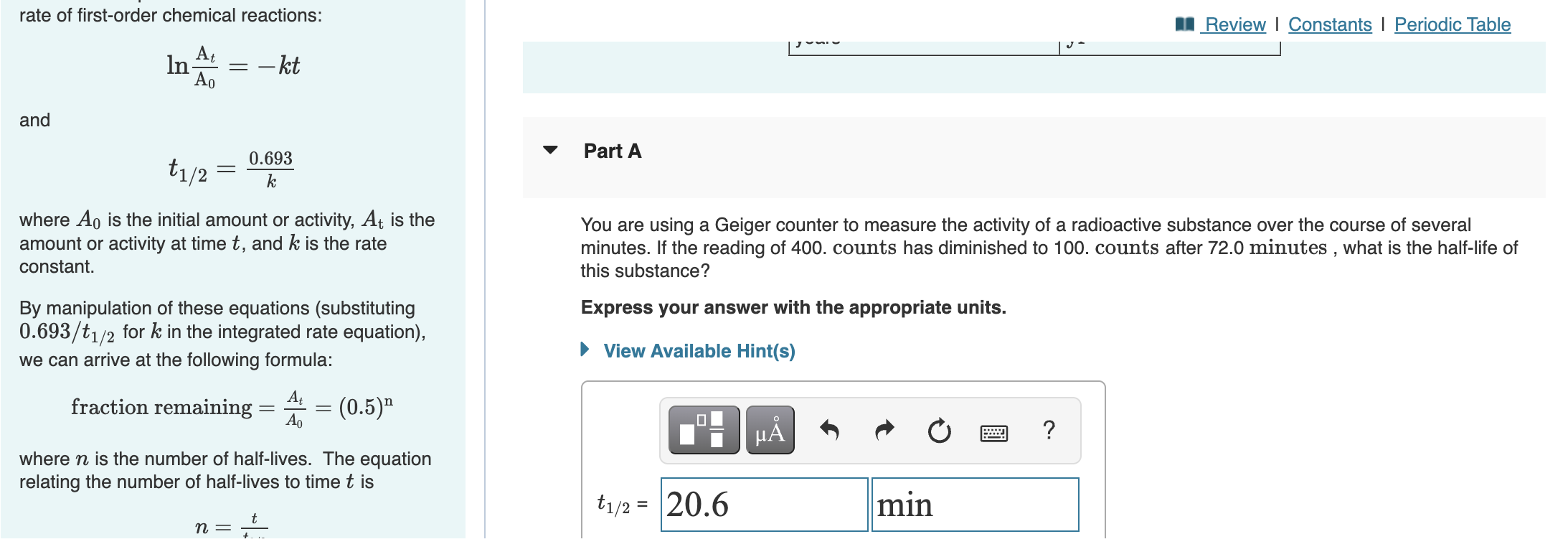
✓ Solved: The rate constant for a certain radioactive nuclide is 1.0 × 10^-3 h^-1 .What is the half-life...
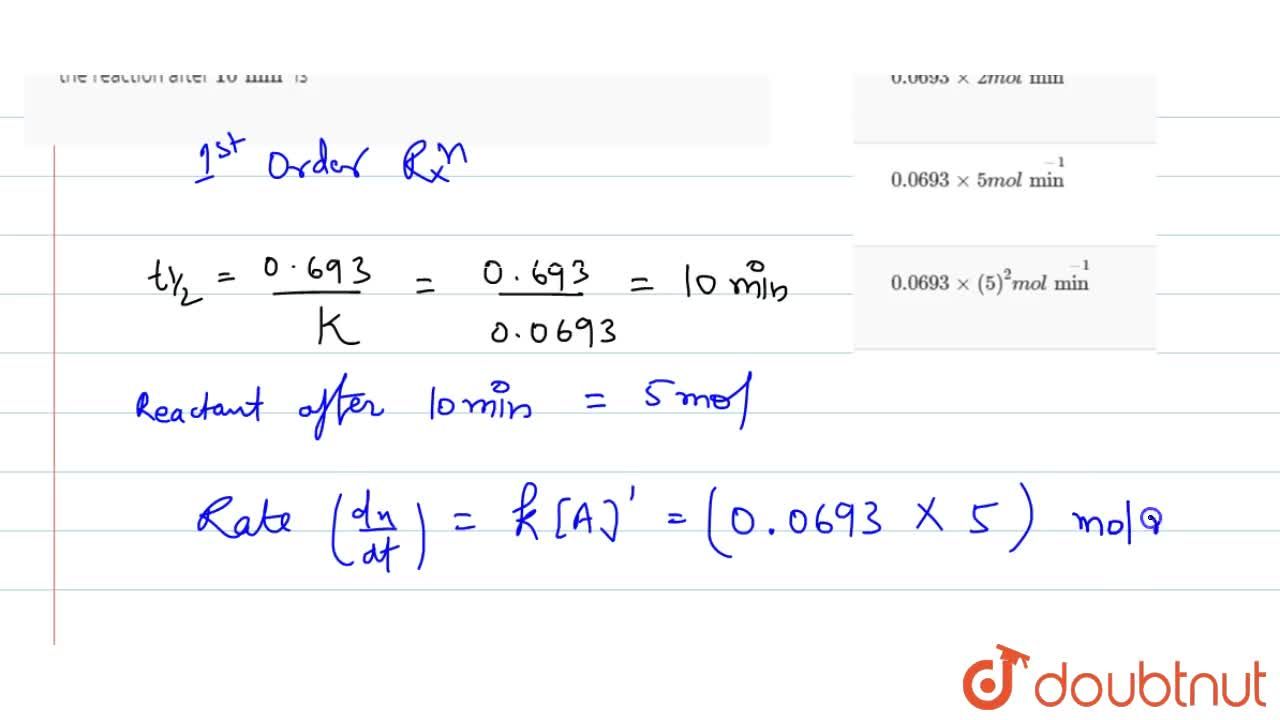
The rate constant of a reaction is 0.0693 min^(-1). Starting with 10 mol, the rate of the reaction after 10 min is

The energy of activation and specific rate constant for a first order reaction at 25^∘ C are 100kJ/mole and 3.46 × 10^-5sec^-1 respectively. Determine the temperature at which half - life of
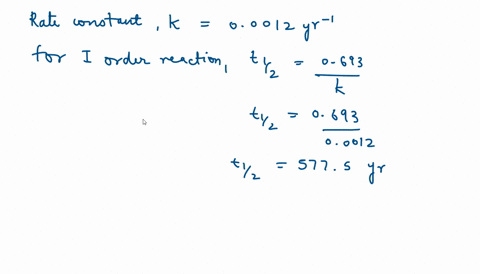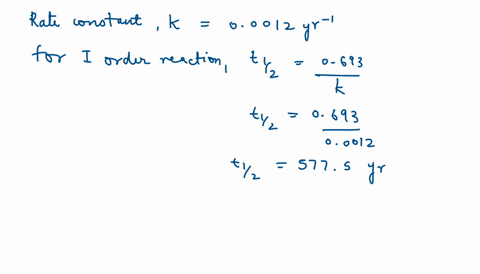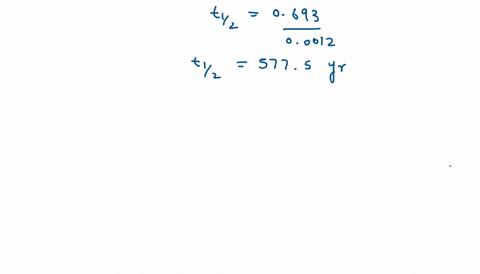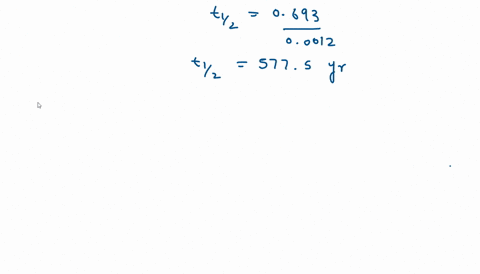
SOLVED:The decomposition of Y is a zero-order reaction. Its half-life at 25^∘ C and 0.188 M is 315 minutes. (a) What is the rate constant for the decomposition of Y ? (b)

The half - life period for a first order reaction is 693 seconds. The rate constant for this reaction would be:

Rate constant of a reaction is 0.0693 min ∧ 1, starting with 10 moles. Rate of reaction after 10 minutes is ???
10. rate constant of first order reaction is 0.0693per minute . calculate the percentage of reaction remaining at the end of the 60 minute.(with detail solution )

Solve this: Q 5 In a first order reaction, the rate constant is 0 693 hr-1 The half-life - Chemistry - Chemical Kinetics - 12322113 | Meritnation.com

OneClass: Half-life equation for first-order reactions: t1/2= .693/k where t1/2 is the half-life in s...

![Solved t1/2 = 1/k[A]. t 0,693/ 1/2 k -2 What is rate the | Chegg.com Solved t1/2 = 1/k[A]. t 0,693/ 1/2 k -2 What is rate the | Chegg.com](https://media.cheggcdn.com/media/560/5609f2c5-63ae-4aef-86f7-fb89896e383e/phpXExk27.png)