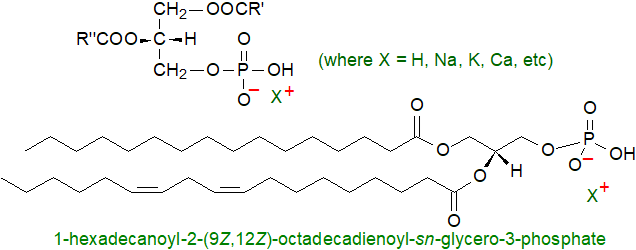
Selective preparation of bio-based high value chemical of p-tolylaldehyde with Cr(OH)3@Fe3O4 catalyst | SpringerLink

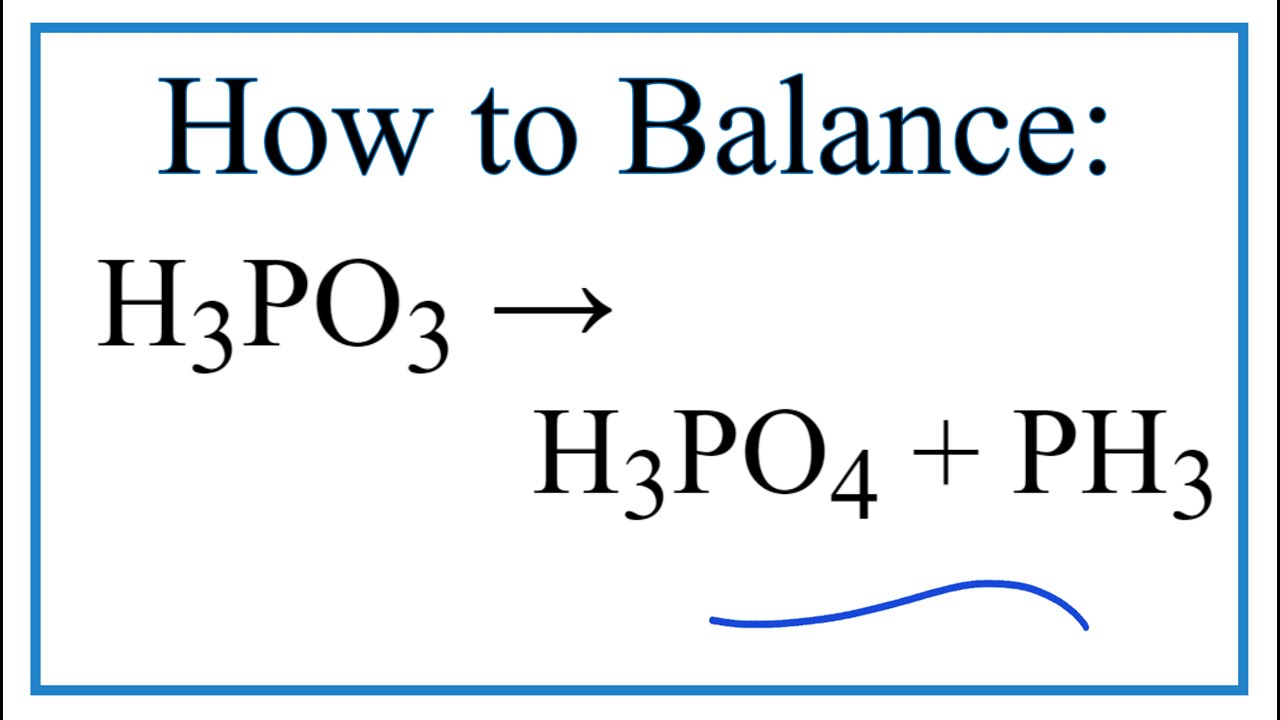
Why is phosphorous acid H3PO3 and not P(OH)3 - which should be more accurate as per the molecule structure? - Quora
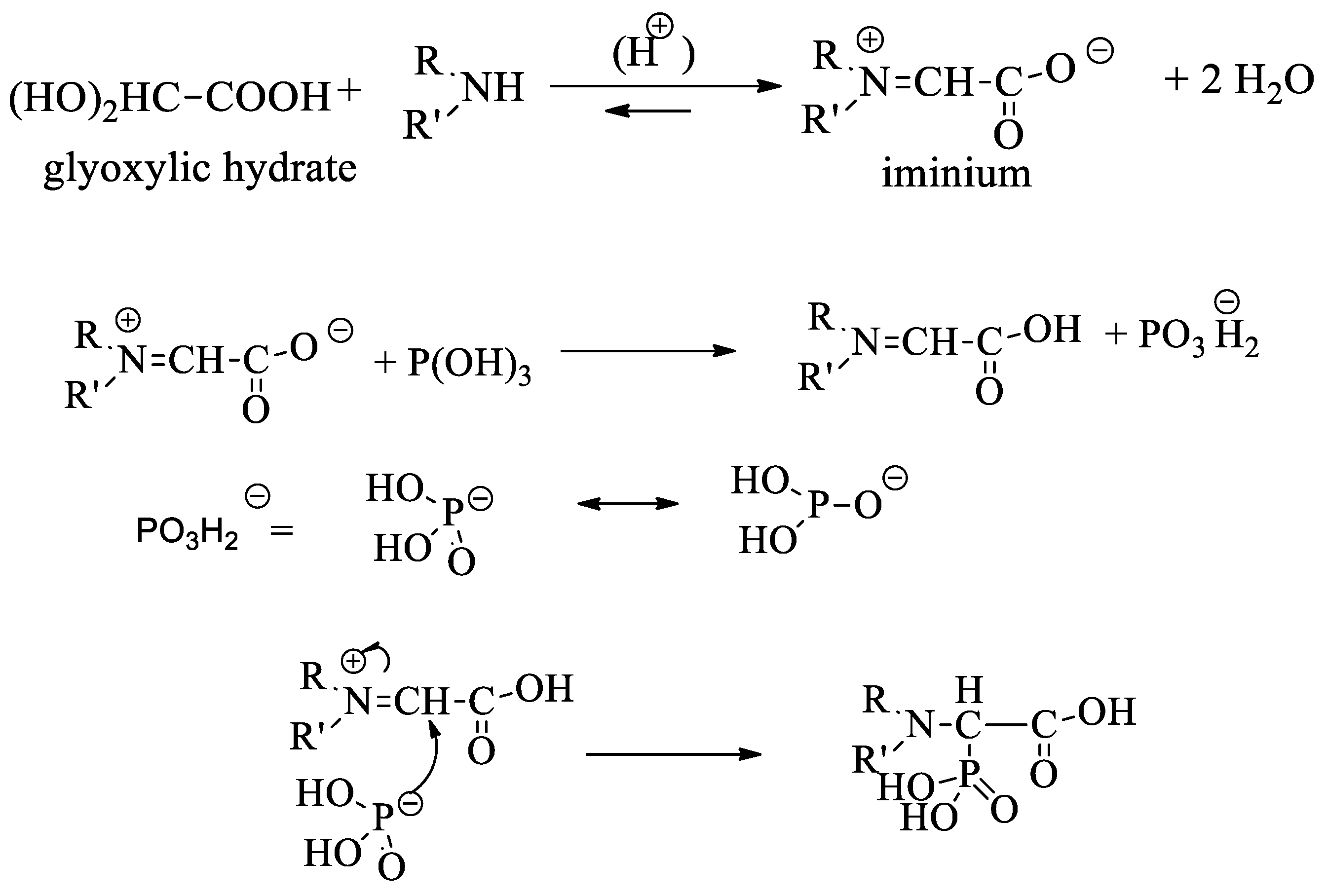
Organics | Free Full-Text | MCR under Microwave Irradiation: Synthesis in Water of New 2-Amino-bis(2-phosphonoacetic) Acids

Highly Efficient Low-Concentration Phosphate Removal from Effluents by Recoverable La(OH)3/Foamed Nickel Adsorbent | ACS Omega
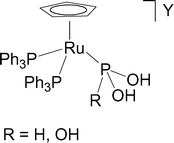
Stabilization of the tautomers HP(OH)2 and P(OH)3 of hypophosphorous and phosphorous acids as ligands - Dalton Transactions (RSC Publishing)

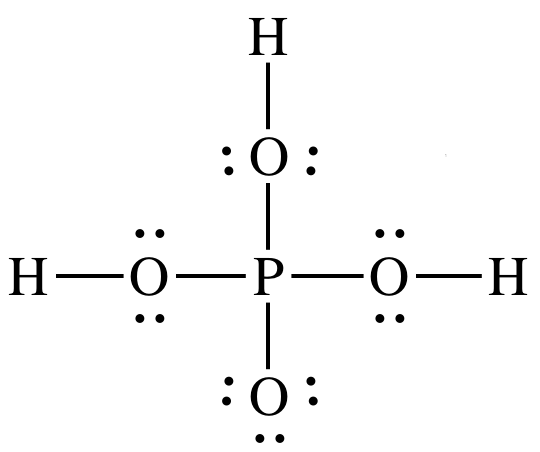
Phosphorous acid, H_3PO_3, has the structure (HO)_2PHO, in which one H atom is bonded to the P atom, and two H atoms are bonded to O atoms. For each bond to an
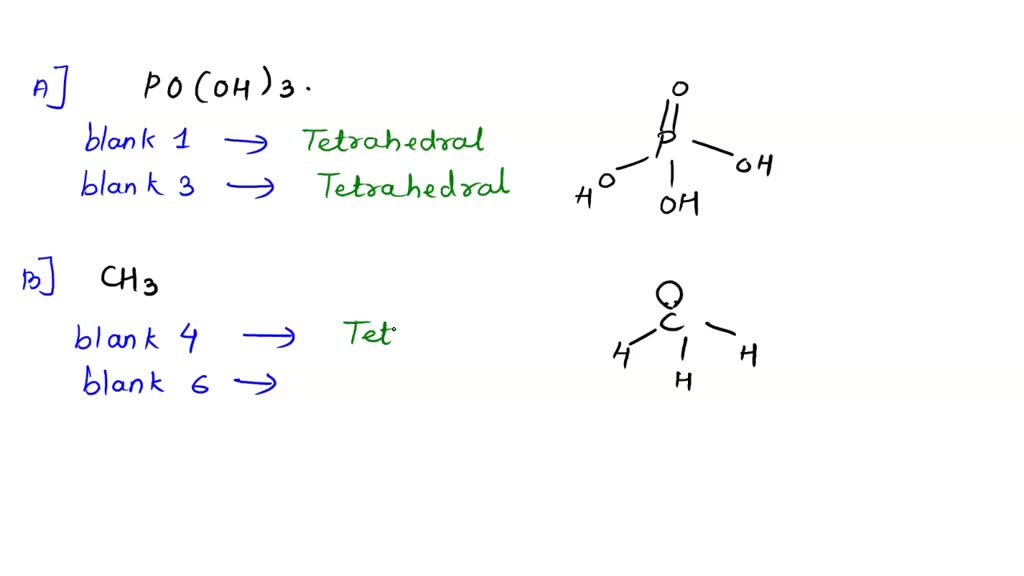
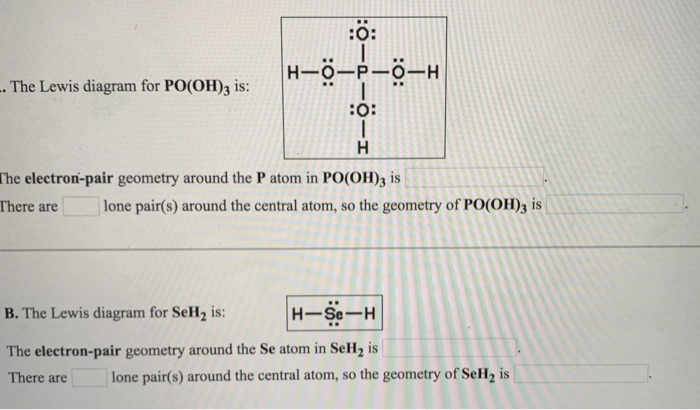
SOLVED: A. The Lewis diagram for PO(OH)3 is: The electron-pair geometry around the P atom in PO(OH)3 is fill in the blank 1. There are lone pair(s) around the central atom, so
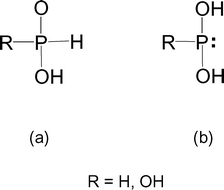
Stabilization of the tautomers HP(OH) 2 and P(OH) 3 of hypophosphorous and phosphorous acids as ligands - Dalton Transactions (RSC Publishing) DOI:10.1039/B510479C
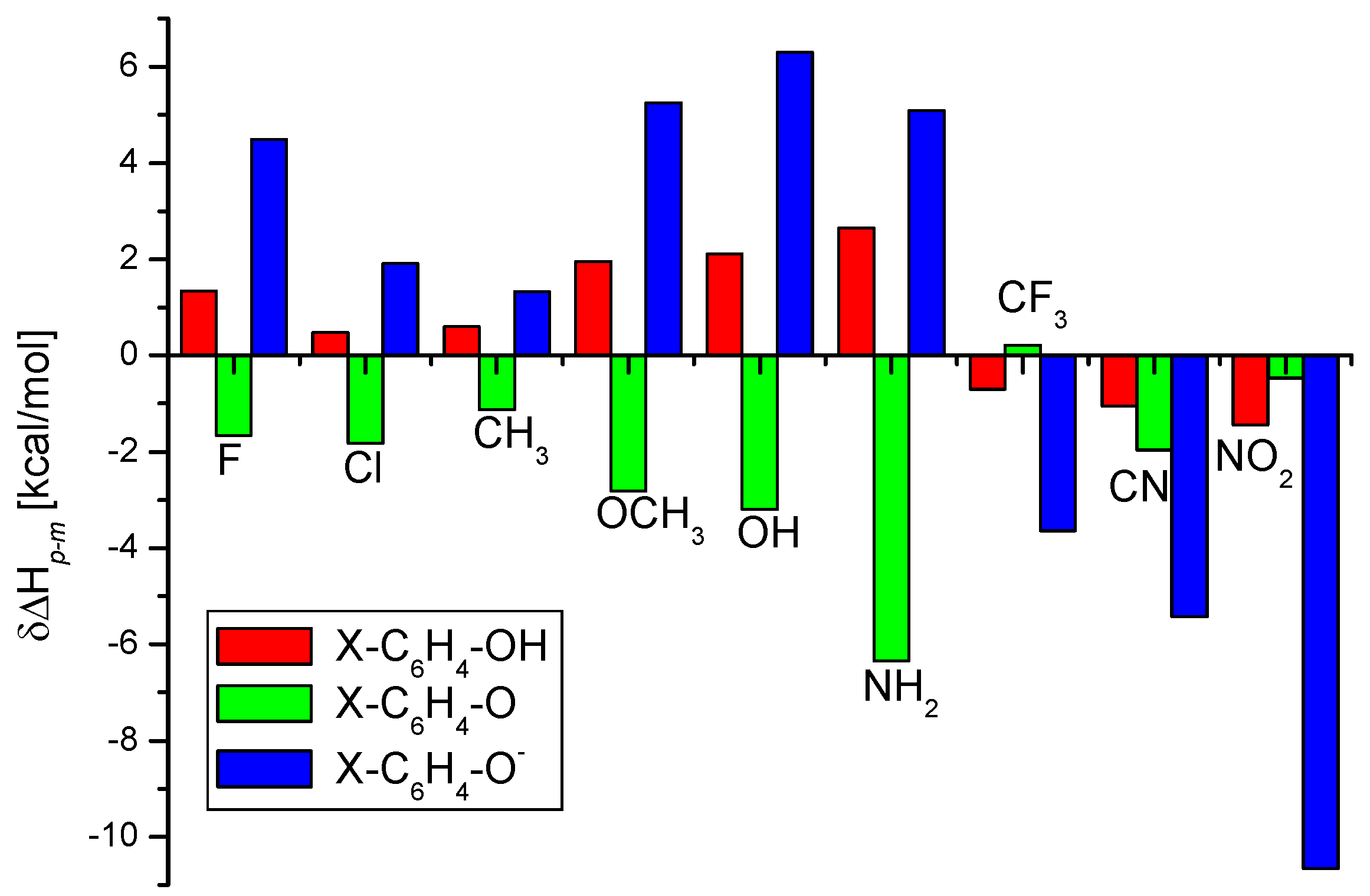
IJMS | Free Full-Text | The O-H Bond Dissociation Energies of Substituted Phenols and Proton Affinities of Substituted Phenoxide Ions: A DFT Study