![p-Toluenesulfonic acid-catalyzed one-pot synthesis of 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitriles under neat conditions - ScienceDirect p-Toluenesulfonic acid-catalyzed one-pot synthesis of 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitriles under neat conditions - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1631074813002646-sc2.jpg)
p-Toluenesulfonic acid-catalyzed one-pot synthesis of 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitriles under neat conditions - ScienceDirect

Write the detailed mechanism for the following reaction. Give all elementary steps. (TsOH = p-Toluenesulfonic acid) | Homework.Study.com
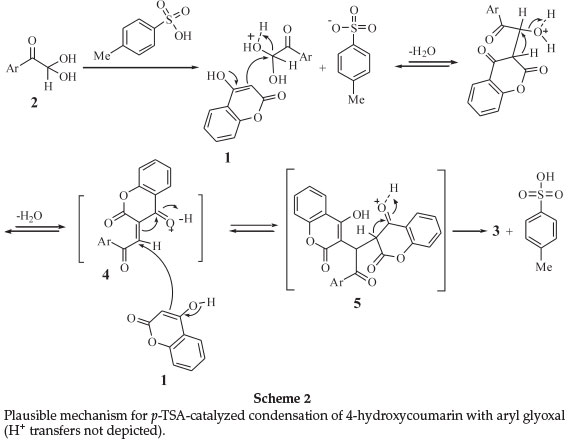
A facile and practical p-Toluenesulfonic acid catalyzed route to dicoumarols containing an Aroyl group

Detailed Characterization of p-Toluenesulfonic Acid Monohydrate as a Convenient, Recoverable, Safe, and Selective Catalyst for Alkylation of the Aromatic Nucleus | The Journal of Organic Chemistry

p-Toluenesulfonic acid mediated hydroarylation of cinnamic acids with anisoles and phenols under metal and solvent-free conditions - ScienceDirect

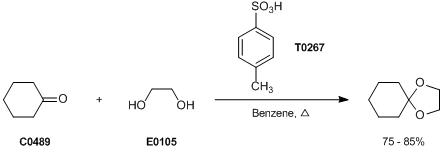
Catalytic systems containing p-toluenesulfonic acid monohydrate catalyzed the synthesis of triazoloquinazolinone and benzimidazoquinazolinone derivatives | SpringerLink

Catalytic systems containing p-toluenesulfonic acid monohydrate catalyzed the synthesis of triazoloquinazolinone and benzimidazoquinazolinone derivatives | SpringerLink
Sciencemadness Discussion Board - ortho nitration of p-toluenesulfonic acid(QUESTIONS) - Powered by XMB 1.9.11
![Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium](https://www.degruyter.com/document/doi/10.1515/hc-2020-0128/asset/graphic/j_hc-2020-0128_ga_001.jpg)
Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium

p‐Toluenesulfonic Acid Promoted Annulation of 2‐Alkynylanilines with Activated Ketones: Efficient Synthesis of 4‐Alkyl‐2,3‐Disubstituted Quinolines - Peng - 2010 - European Journal of Organic Chemistry - Wiley Online Library
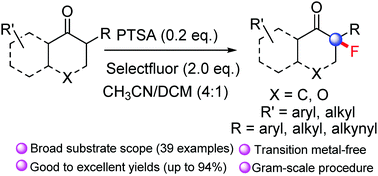
p-Toluenesulfonic acid catalysed fluorination of α-branched ketones for the construction of fluorinated quaternary carbon centres - Chemical Communications (RSC Publishing)

p-Toluenesulfonic acid functionalized imidazole ionic liquids encapsulated into bismuth SBA-16 as high-efficiency catalysts for Friedel–Crafts acylation reaction - Dalton Transactions (RSC Publishing)
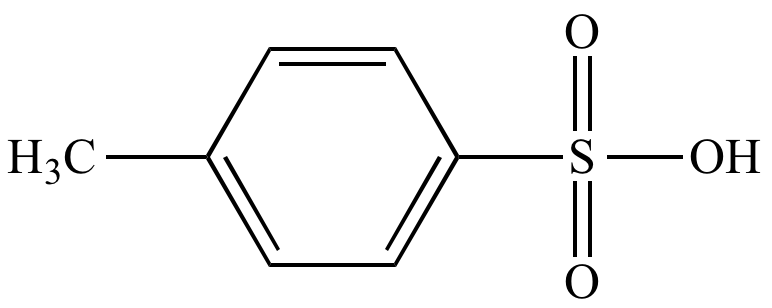
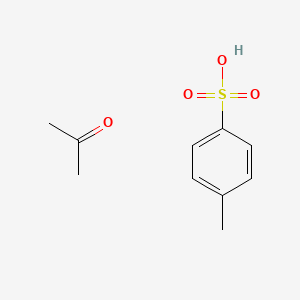
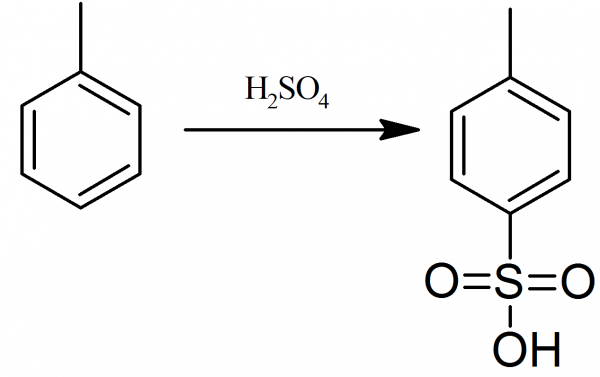
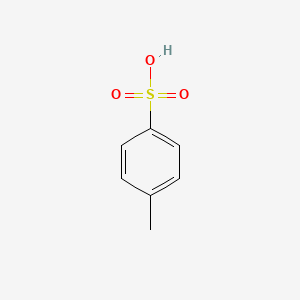
Illustrated Glossary of Organic Chemistry - Toluenesulfonic acid (p-toluenesulfonic acid; TsOH; p-TsOH)

Supported p-Toluenesulfonic Acid as a Highly Robust and Eco-Friendly Isocyanide Scavenger | ACS Combinatorial Science