FDA authorized molecular point-of-care SARS-CoV-2 tests: A critical review on principles, systems and clinical performances - ScienceDirect

Roche Diagnostics USA on Twitter: "@Vswilliamson Thanks again for the RT! Have a great Tuesday." / Twitter
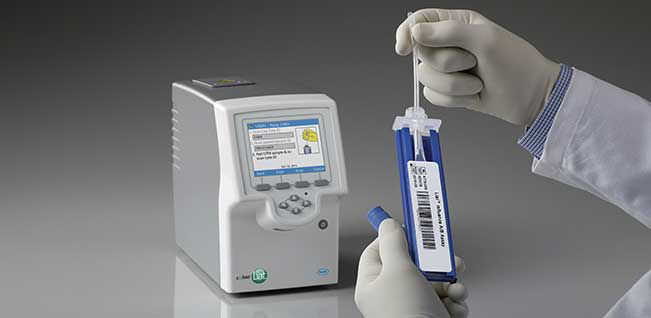
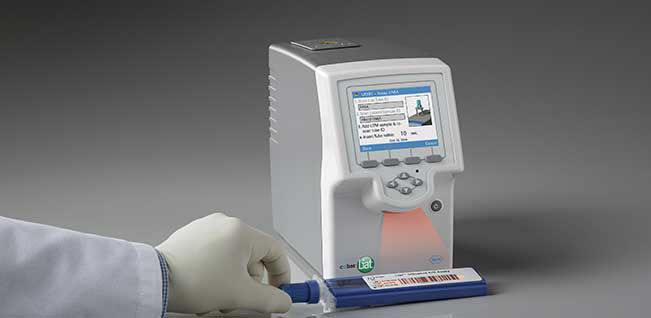
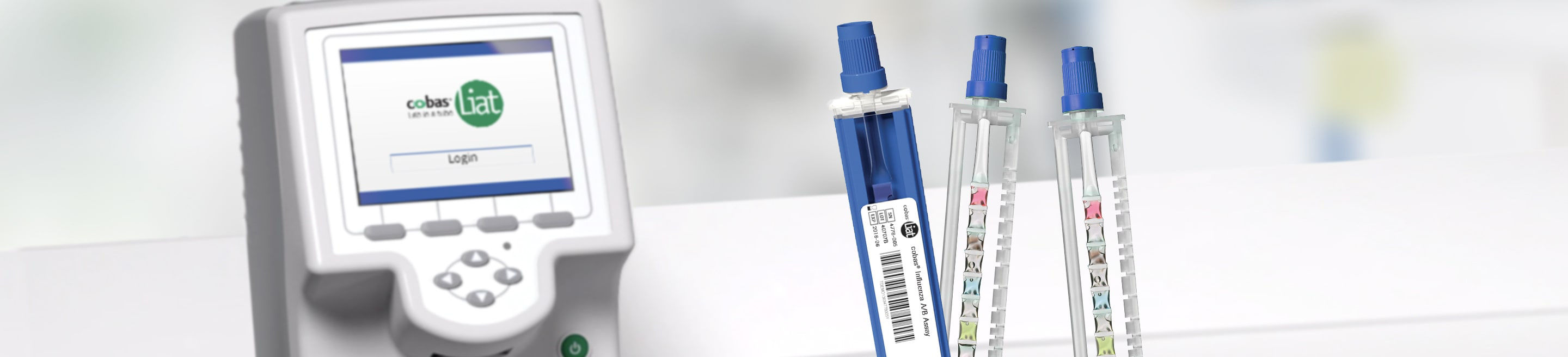
Quick Reference Instructions cobas® SARS-CoV-2 & Influenza A/B Nucleic acid test for use on the cobas®Liat® System For u
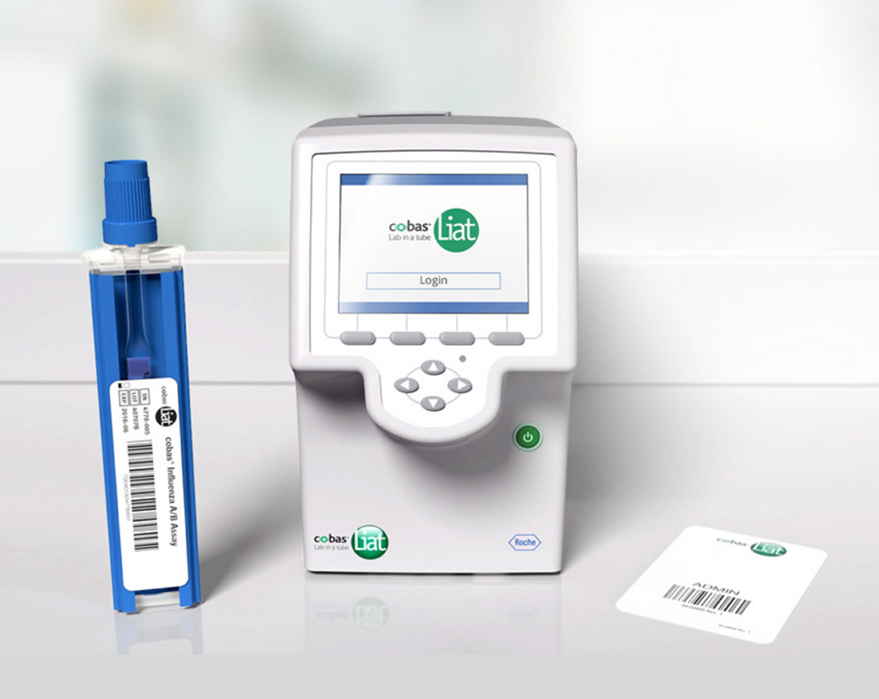
KEI Research Note 2020:1 Government funding related to the cobas Liat Analyzer PCR testing device - Knowledge Ecology International